
This
is the first of what is hopefully going to
become a regular section in our magazine.
The articles will cover in general terms aspects of the science of
horticultural production. For those who are
familiar with this subject this will serve as a
refresher, and for others an insight of what
might be occurring in your crop. In this first
article we look at the subject of pH which is
often over looked and underestimated in its
effect on plants.
The pH story
pH is
the term used to describe or measure the acidity
or alkalinity of a soil or solution. The full
measure or scale is 0-14 with 0-7 being acidic
and 7-14 alkaline. 7 being in the middle of the
scale is neutral, 0 being the extreme in acidic
and 14 the
extreme in
alkaline or caustic. In agricultural soils or
waters the range is usually between 5 and 8.5.
In scientific terms pH is a measure of the concentration of hydrogen
ions (H+) with the higher the concentration
being reflected by the stronger acidic values,
ie the lower the pH reading.
Most people
only measure the pH of the soil growing the
crop, however in horticulture where regular
irrigation is part of the production process,
water pH can play a significant role. Plants
don't just grow in soil, they grow in a solution
combination of soil, water, air and cultural
additives (namely fertilisers) and it is the
resultant combination of pH that we need to be
paying the attention to.
As an example
if we have a soil with a pH of 6.5 a water
source with a pH of 7.6 and an alkaline
fertiliser is used then |
|
|
Back to basics...
the resulting soil
solution could easily remain alkaline, which is
not ideal for maximising horticultural cropping
potential. For nearly all of the crops we grow
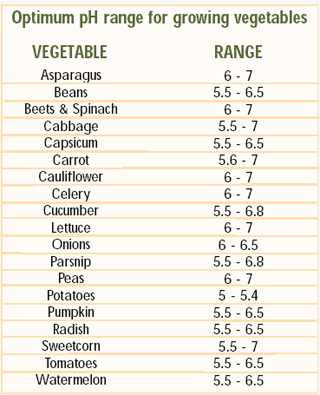
the desirable range for pH is 6 - 6.5 (see table
below for desirable crop pH ranges).
Another significant factor for trying to
keep your combined soil solution in this range
is that at these levels all of the necessary
nutrients are available to the plant. If our
soil solution becomes more acid or alkaline the
availability of our plant foods is decreased or
in some extreme cases can be made available but
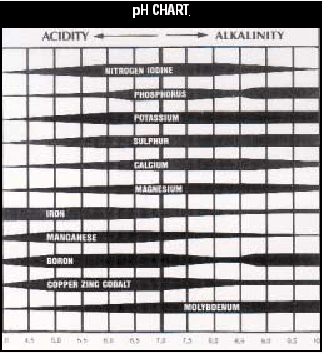
in toxic quantities. Looking at the pH chart on
the left, you can see the widths of the bars
indicating availability of the nutrients at the
various pH levels, wider being more available
and narrowing being less available. If you draw
a line down the 6.5 value you can see that
nearly all nutrients are of their maximum
availability - TOGETHER. Welcome to the
balancing act.
Balancing takes
experience but by getting it right you can
prevent a lot of problems from developing and
make it that much easier to maximise crop
potential. In summary pH is rarely a fixed point
with any farming operation so it is wise to set
up procedures where this value is monitored at
regular intervals within a cropping season. The
main points again:
 Measure of acidity
(0-7) or alkalinity (7-14).
Measure of acidity
(0-7) or alkalinity (7-14).
 Crops grow in the combined soil solution of
soil, water and additives.
Crops grow in the combined soil solution of
soil, water and additives.
 Each crop has its own desirable pH range.
Each crop has its own desirable pH range.
 pH can influence the availability and uptake of
nutrients.
pH can influence the availability and uptake of
nutrients.
 |
|